too much dha during pregnancy
 5 Reasons You Should Not Take Too Much DHA During Pregnancy? HealHealth.in
5 Reasons You Should Not Take Too Much DHA During Pregnancy? HealHealth.inWarning: The NCBI website requires JavaScript to operate. Excess Omega-3 Consumption of fatty acids by mothers during pregnancy and breastfeeding Shorter Life practices Span and ABR abnormal in older descendantsM. W. ChurchaDepartment of obstetrics and gynaecology, Wayne State University School of Medicine, Detroit, MI 48201, USAK-L. C. JenbDepartment of Nutrition and Food Science, Wayne State University of DetroitJ I. AnumbacDepartment of Physiology, Wayne State University School of Medicine, Detroit, MI 48201, USAD. A. JacksoncDepartment of Physiology, Wayne State University School of Medicine, Detroit, MI 48201, USAB. R. AdamscDepartment of Physiology, Wayne State University School of Medicine, Detroit, MI 48201, USAJ. W. HotraaDepartment of Obstetric Gynaecology, Wayne State University School of Medicine, Detroit, MI 48201, USAAbstractConsuming omega-3 fatty acids during pregnancy and breastfeeding is beneficial for fetal and child development and could reduce the incidence and severity of premature births by prolonging pregnancy. Consequently, the supplementation of maternal diets with large amounts of ω-3 FA is gaining acceptance. However, both overcoming and sub-suplementation with ω-3 FA may harm the development of the offspring. Adverse fetal and neonatal conditions in general can improve age-related neuronal degeneration, shorten the life period and cause other adult disorders. Hypothesis that maternal overnutrition and undernutrition with ω-3 FA would shorten the lifetime of offspring and improve neuronal degeneration in adulthood. To test these hypotheses, female Wistar rats were randomly assigned to one of the three dietary conditions from day 1 of pregnancy throughout the period of pregnancy and breastfeeding. The three diets were Control ω-3 FA (ω-3/ω-6 ratio ~ 0.14), Excess ω-3 FA (ω-3/ω-6 ratio ~ 14.5) and Deficient ω-3 FA (ω-3/ω-6 ratio ~ 0% ratio). When possible, a male and female descendant of each chamber was evaluated for the period of life and sensory/neural degeneration (n=15 liters/group). The Excess descent had a shorter life compared to its Control and Deficient cohorts (mean±SEM=506±24, 601±14 and 585±21 days, p≤0.004) when the study ended the postnatal day 640. Excess descendence had a higher incidence of presbicusis than the Control and Deficient groups (33.3, 4.3 and 4.5%, p=0.011) and a persistence of other sensory/neurological abnormalities and lower body weights in the old age. In conclusion, the overnutrition or imbalance of the FA ω-3 during pregnancy and breastfeeding had adverse effects on the useful life and sensory/neurological function in adulthood. The adverse results in the offspring were probably due to an "annual toxicity" during the fetal and/or neonatal development that programmed them for lifelong health disorders. The health implication is that eating or managing large amounts of ω-3 FA during pregnancy and breastfeeding seems inadvisible due to adverse effects on offspring.1. IntroductionOmega-3 fatty acids (ω-3 FA), especially docosahexanoic acid (DHA) and eicosapentanoic acid (EPA) of fish oil, have received much attention recently as prenatal and postnatal treatments. Dietary supplementation ω-3 FA can increase birth weight and prolong pregnancy, thus reducing the incidence and severity of premature births and low birth weights [,]. FA NIC-3 in breast milk or fortified child formulas can improve neurocognitive and visual development during the first year of life compared to infants who receive child formula without supplements from the NIC-3 FA [,]. Therefore, increasing doses of FA are being recommended for pregnant women and nursing babies to promote the health of premature development, low birth weight and even normal babies [,]. Not all the researches match these findings, however, as there are gross evidence that diets rich in ω-3 FA can be harmful to the fetus and the neonate. Several human studies have reported a decrease in gestational length and/or delay in fetal growth [,,,,] and an increase in infant morbidity [] of high consumption of fish oil by the mother during pregnancy. The adverse effects of the high consumption of ω-3 FA by the infant formulas of fortified drink with ω-3 FA may include a growth of the reduced body [,], the circumference of the reduced head [], the decrease in the levels of arachidonic acid of blood [,] and the decrease of verbal abilities [,]. A recent clinical trial reported that fish oil supplements taken by pregnant mothers and infants can reduce the infant body mass index []. Animal studies also report adverse effects. Prenatal and/or postnatal dietary supplementation with large amounts of ω-3 FA or a high proportion of NIC-3/ω-6 FA may result in a reduction in birth weight, a deterioration of postnatal growth, increased prenatal and postnatal mortality, a decrease in the sizes of the brain, a decrease in AA levels, abnormal brain structure/function [,,,,,,,,,,,] and a structure/function Studies that use the auditory response of the brain (ABR), a brain development measure and sensory function, found that high levels of dietary supplement ω-3 FA in pregnant rats and infants caused the offspring to have prolonged neuronal transmission time [,], delayed acoustic reflexes of the beginning [,], reduction of hearing acudeza [], and brain alteration []. The harmful effects were caused similarly by dietary deficiency ω-3 FA. These effects included poor visual function [,], learning deficits, brain weight reduction and/or alteration of the composition of nerve FAs [,,,,] and ABRs indicating a faster hearing nervous system in old age []. Adverse fetal and neonatal conditions can improve age-related neuronal degeneration, shorten the life period and cause other disorders of adult appearance, such as type II diabetes, obesity, hypertension and coronary disease. This is known as the Barker or the Hypothesis "Fetal Programming" []. No one has investigated the possibility that nutritional toxicity from high levels of diet ω-3 FA or a high ratio ω-3/ω-6 can cause fetal programming of disorders of active adults, with the exception of studies of our group that found an abnormal neurological function [] and altered the composition of body fat in young adult rats born to preys that receive diets rich in ω-3 FA breastfeeding. Only an animal study has investigated the effects of lifelong prenatal essential FA deficiency and has found a faster aging brain in old age as shown by ABR []. Therefore, it is clinically important to undertake further research to fill the knowledge gaps on the potentially long-term harmful effects of the excess and deficiency of the perinatal FA. We hypothesize, therefore, that maternal consumption of excessively rich or deficient diets in ω-3 FA during pregnancy and breastfeeding would shorten the life of the offspring and improve neuronal degeneration in adulthood as demonstrated by the improved aging of ABR. ABR is a sensitive measure of development and brain and sensory function [,]. It is also an indirect measure of cerebral myelation []. Mother rats were treated during pregnancy and breastfeeding because the brain development of rats during early postnatal life is similar to that in the human brain during late pregnancy [] and because human mothers can take ω-3 FA supplements during pregnancy and breastfeeding. In previous studies, our condition of excess of FA ω-3 caused delay in postnatal growth, abnormal ABR in the offspring of 24-day rats [,] and a persistence of some ABR abnormalities in young adulthood []. The current study followed these descendants to adult old age to assess some long-term consequences of their treatments.2. Methods2.1. Diets and Animal Numbers Wayne State University's Animal Research Committee approved the procedures for this study. Institutional and NIH guidelines were followed. Our procedures are detailed in other places [,]. Briefly, 10-week-old female Wistar rats were individually paired with male Wistar rats (formerly Hsd:WI rats Wistar, Harlan Sprague Dawley, Inc., Indianapolis, IN 46250, USA). The presence of a sperm cap was designated as the first gestational day. Females were placed in separate cages of polycarbonate (25 × 45 × 20 cm) and randomly assigned to one of the three dietary conditions from day 1 of pregnancy throughout the period of pregnancy and breastfeeding. The three diets were the condition of Control ω-3 FA (ω-3/ω-6 ratio ~ 0.14), the ω-3 FA (ω-3/ω-6 ratio ~ 0% ratio) and Excess ω-3 FA condition (ω-3/ω-6 ratio ~ 14.0). The Control diet contained 7% soy oil. The ω-3 FA deficient diet contained 7% safflower oil instead of soy oil. The Excess ω-3 FA diet contained 7% menhaden oil (a type of fish oil) instead of soy oil. Our justification for these dosage selections is detailed in other places [,]. We believe that this is an excess of diet ω-3 FA because it had a ω-3/ω-6 ratio that was 100 times what is suitable for pregnant rats [] and infants and because our diet of 7% fish oil is consistent with other studies that report adverse effects for development [,] All diets were formulated according to the ideal AIN-93G rules that have determined that the ratio is ω-3-6 Fish oil was selected for the Excess diet due to its use in clinical studies. Soybean and glow oils were selected in the Control and Deficiency diets because humans consume them commonly and are used in animal studies. We use fatty acid profiles that naturally occur from fish, soy and safflower oils; nothing artificially altered. Diets were prepared by Dyets Inc (Bethlehem, PA 18017, USA). The three diets contained tertiary butilhidroquinone (TBHQ) because this preservative restricts oxidation [,]. Diets were stored at cooling temperatures and a fresh diet was provided twice a week to protect against oxidation. Each diet provided 3.96 kcal/g. Recently, [,] the detailed composition of each diet, cattle raising, maternal results and offspring were published. The weaning occurred on postnatal 21 (PND 21). After PND 24, the descendents were sangly housed and switched to 5001 Rodent Diet (PMI Nutrition International, Richmond, IN 47374, USA). Our initial studies began with the Control, Deficient and Excess groups that had numbers of 23, 31 and 22 respectively [–]. Due to the decrease in financial resources, we have cultivated our animal colony to 15 liters/group for the current study retaining the oldest litres. A male offspring and a female per litre were used when possible. For control, the deficient bunk beds and excess, the number of male and female offsprings were 12+13=25, 11+13=24 and 13+12=25 for the life period and the result variables of body weight (total N=74 offspring). ABR result variables were evaluated at ~16 months of age. By this age, the size of the animal colony had decreased because several animals died from natural causes. Consequently, the ABR study had numbers of 14, 13 and 9, respectively. The respective number of male and female offspring was 12+11=23, 9+13=22 and 8+7=15 for ABR result variables (total N=60). 2.2. Length of life, body weights and necropsies For the portion of the life of our study, the animals were allowed to die naturally. The exception was when an animal seemed to be suffering to an unacceptable degree as determined by the veterinarian. This usually meant that the animal was too weak to eat or drink and was losing weight quickly. In the first sign of significant weight loss (~10% decrease), an animal would be placed in prepared soft foods moisturizing the food bags in the water. Often, an animal would respond with weight stabilization and would stay for weeks with this procedure. When the condition of an animal deteriorated to the point that it was clear that the animal would not survive longer than a few days, it was humanly sacrificed by inhalation of carbon dioxide followed by bilateral pneumothorax. Life was measured as the age of the animal at the date of its sacrifice or the date of its natural disappearance. The study of the duration of life was completed for any animal that reached the age of 21 months (640 days). By this age, virtually all the children of Excess had died and it was clear that the descendants of Control and Deficiency were not different in their survival rates. The study was completed at 21 months of age to preserve our limited financial resources. Animals who still live at 21 months of age were assigned at a age of 640 days to allow later data analysis by the Kaplan-Meier survival curves and the Log Rank tests. Birth weights, neonatal weights and weights of young adulthood have been previously published [–] and are briefly described here. From the age of three months, each animal was weighed at intervals of three months until the age of 21 months (640 days). If an animal died before 21 months of age, his weight was also recorded on the day of death. His mortal weight was also incorporated into each subsequent weighing session as a substitute for missing data. This allowed us to carry out an analysis of repeated measures of differences in body weight data. Although it is not part of the original protocol, several animals (4 Control, 3 Deficient and 9 Excess of offspring) who died in old age were given necropsies by a veterinarian to help determine the cause of death. The kidneys, livers and lungs of the animals were brusquely inspected histologically by pathology. Blood samples were collected by the veterinarian when it was clear that these animals were approaching death. Blood samples were analyzed by the University's Animal Resources Department of Laboratory. The mummary adenomas (tumores) were raised for all animals. The choice of bodies surveyed was influenced by previous studies of the useful life of rats [,].2.3. Procedure ABR2.3.1. ABR Recordings When possible, a man and a pup/litter woman were randomly selected to test in the current study. Using male/feminine littermato pairs allowed to evaluate sex-dependent differences and control for the lowest effects by limiting the number of puppies tested from any litre. The rat descendants were initially tested with ABR in PND 24 and the results of the tests were previously reported [,]. These same offspring were later retested as young adults and those results were reported in another article []. These animals were older adults, 16 months old (~480 days) at the time of the ABR test reported in the current article. ABR rat is fully mature and stable about 70 to 100 days old [] and age-related hearing loss (presbycusis) usually does not occur in rats up to about 17 months []. Our ABR procedure is detailed in other places [,]. Before the ABR recording, each animal received 100 mg/kg of anesthetic cetamine (e.g.). Ketamine influences the delays and amplitudes of ABR, but the effects are lower and the quality of ABR is excellent []. Rectal temperature was monitored because temperature can influence ABR [] (Model 43TD, Yellow Springs Instruments Co., Yellow Springs, OH 45387, USA). A circle-water heating pad was used to regulate and maintain the normothertermia by raising or lowering the circulating water temperature (Model TP500, Gaymar Industries, Orchard Park, NY 14127, USA). The ABR was recorded in a differential way between two electrodes of subcutaneous platinum needles E-2. The active electrode was inserted into the vertex, the reference electrode under the left ear, and the electrode of the ground under the right ear. The potentials evoked were collected by a biological-logic navigator and amplified 300,000 times with a digital bandpass of 300-3000 Hz (Bio-logic Corp, Mundelein, IL 60060, USA). The electrode impedances ranged from 0 to 9 k ́Ω. At least 256 responses were mediated. The recordings were made in a double wall sound attenuation chamber (Allotech, Inc., Raleigh, NC 27603, USA). Open field tone pyps in the ascending order of 2000 Hz, 4000 Hz, 8000 Hz and 16000 Hz were delivered through a TDH-39P headset located in front of the animal with up/fall time = 0.5 mss, plateau = 1.0 mss, polarity = alternating, repeat rate = 19,0/seg, and stimulus intensity = 15 to 100 2.3.2. The ABR thresholds (hearive hold) were determined by the boundary method [,]. Here, serial ABRs were collected to a range of stimulus intensities starting at 100dB, dropping to 80, 60, 50, 40, 35, 30, 25, 20 and 15 dB when the ABR threshold was reached and passed. To establish the ABR threshold more precisely, 2 and 3 dB changes were evaluated at stimulus intensity levels around the ABR threshold (according to visual detection) and multiple ABR tracks (2 to 5) were collected at each almost resistant intensity level. Threshold was defined as the lowest intensity to obtain an ABR component with reliable score. An experimenter, who was "blind" as to the treatment condition of each animal, noted the ABR thresholds. A second experiencer checked the score threshold for reliability purposes. 2.3.3 ABR Latency Intensity Profiles APR Latency Intensity Profile (L-I) can help determine whether a subject's hearing loss is a conductive hearing loss (CHL) or sensorineural hearing loss (SNHL). A subject with a CHL would have a high ABR threshold and an L-I profile that moves up and parallel to the normal curve. A subject with a SNHL will also have a high ABR threshold, however, P2 latences will normally be normal or almost normal in response to strong stimulus intensity but gradually curve upwards from the normal range while stimulus intensity decreases []. In rodents, one typically uses the ABR P2 wave to derive the latency intensity profile of an animal (L-I) because the P2 rodent wave is the largest wave and the last wave to disappear as the sound stimulus is diminished []. P2 latency was measured in the positive peak of this wave form at each level of stimulus intensity [].2.3.4. ABR amplitude profiles The width of the P2 wave was used as an index of the maximum amplitude of the ABR because the P2 wave of the rodent is the largest wave and the last wave to disappear as the intensity of the sound decreases. The P2 amplitude was measured from the positive peak of the P2 wave to the subsequent negative trough (not labeled N2). This was done at each level of stimulus intensity to obtain amplitude intensity profiles (A-I). While ABR amplitude is a neuronal synchronization function and the number of triggered neuronal units, the ABR range can provide diagnostic information about these neuronal functions. 2.3.5 ABR Latencias (neural transmission time) ABR is a series of action potentials and post-inoptical potentials. The ABR rat consists of four components (marked P1 to P4) that occur within 6 ms of stimulus start [,,,]. Although the neurogenerators of the ABR of the rat have not been determined, in the mouse they reflect the neuronural activity mainly of the auditory nerve (P1), the cochlear nucleus (P2), the upper olive complex (P3), and the lateral and/or lower colliculus (P4) []. The latency of each ABR component was measured as time from the triggering of the computer headset to the positive peak of a wave, including a 0.3 m acoustic transit time between the headphone and the animal's pine. Two experimenters, who were "blind" in terms of the treatment condition of each animal, noted the delays of the ABR P1, P2, P3 and P4 waves. When the scorers do not agree (actually), the scores are averaged. The primary result variable was P4 latency, a measurement of neuronal transmission time along the auditory nerve and the auditory pathway of the brain inclusively caused by the 100 dB stimuli. Side results were delays of individual ABR waves and interpeak P1 latency to P4 (P1–P4 IPL). P1-P4 IPL measures the brain stem part of neuronal transmission by excluding hearing transmission time from the nerve. 2.4. Data Analysis Kaplan-Meier survival curves and Log Rank tests were used to analyze survival data (lifetime). The Log Rank test compares multiple survival curves simultaneously. Although we predicted that over and underdeficient diet treatments would negatively affect the outcome of offspring, the use of directional probability levels was not necessary. The statistical significance criterion was p≤0.05. Analysis of variances (ANOVA) assessed the statistical importance for body weight and ABR variables. Because there is interest in gender differences, male and female lymphocytes are considered individual measurement units. For the body weight variable, a three-way ANOVA was used to test the effects of Diet, Sex and Age. For ABR variables, a three-way ANOVA was used to test the effects of Diet Frequency, Sex and Tone (or Intensity). Because the Age and Tone Pipe were internal measures, the Greenhouse-Geisser adjustment was used with the main effects and interactions of this variable. Because each litre was shown in a very balanced way, using only one male and one female of each litre with a rare exception, it was not necessary to control the effects dependent on the litre using the litre as a variable in the statistical design. If an ANOVA indicated a significant effect of diet treatment (p≤0.05), then univariated ANOVAs were used followed by Bonferroni tests to make couple comparisons planned between treatment groups. 2.5. Timeline To summarize the timeline: Treatment diets were given to prey of gestation day 1 a day of lactation/postnatal 21 when puppies were weaned. Weights were collected at birth and at PND 7, 14 and 21 and again from 3 to 21 months at intervals of 3 months. The ABRs were collected in PND 24, ~174 and ~480. The life-span evaluation ended in PND 640.3. Results3.1. Maternity and Birth Results Maternal and birth data were previously reported [,], so these results are only summarized here for the reader's comfort. In short, there were no group differences in gestational length, maternal weight gain, food consumption during pregnancy and breastfeeding, the number of puppies per litre, liter and pup weights at birth, or the age of tooth rash. A trend was observed in the increase in postnatal mortality between birth and weaning for the breeding of Excess and the detachment of pinena was delayed. In PND 24, we will put the pairs of male/female lymphature that were channeled in the course of life/ABR. There was a significant effect for the Diet group by which male and female puppies weighed considerably less than their Control and Deficient cohorts and poor female puppies weighed less than their Control cohorts. 3.2. Life and survival curves show survival curves. The Log Rank test showed a significant difference in the Diet group (2)=13.351, p=0.001. Comparisons between pairs showed that the Excess' offspring had a shorter life than their Control and Deficient cohorts with the respective average life intervals ± SEM of 506±24, 601±14 and 585±21 days (p≤0.004). Deficient control and offspring do not differ (p=0.992). When the study ended at 21 months of age (640 days), the survival rate for the Excess offspring was only 28.0%. In contrast, the rates of survival for control and deficient descent were 68.0% and 66.7%. Females (597±13 days) lived longer than men (570±15 days) and had higher survival rates at the end of the study (76.9% vs. 55.1%): Chi Square (1)=4.822, p=0.028. This difference depends on sex was consistent with diet groups. Adult Excess breeding had a faster decline in its survival curve than its Control and Deficient cohorts. 3.3. The body weights and necropsies show the adult body weights as functions of the Diet group and the age for male and female offspring. There were significant effects for the Diet group: F(2, 67)=6.506, p=0.003 and for sex: F(1, 67)=100.702, p=0.000 but not for the Diet-by-Sex interaction. Post hoc comparisons indicated that the Excess offspring weighed significantly less than its Control and Deficient cohorts (p≤0.01), while the Control and Deficient offspring did not differ between themselves (p=1,000). There were significant effects for the age: F(6, 402)= 88.587, p=0.000 and for the interaction Age-per-Diet: F(12, 402)=6.381, p=0.000 and the interaction Age-by-Sex: F(6, 402)=18.569, p=0.000 but not for the interaction-by-Diet-by-Sex. Top panel: There were no group differences in weights of the male breeding body (mean±SEM) 3 to 12 months old. From 15 to 21 months of age, the Male Excess offspring weighed significantly less than its male cohorts Control. Lower panel: There were no group differences in female breeding weights of 3 to 12 months old. From 15 to 21 months of age, the female Excess was significantly less than its control cohorts and poor. *Excess males different from cohorts Control; †Excess females different Control and Deficient cohorts. proves that there were no Differences of diet group in weights of the adult male child's body from 3 to 12 months old. From 15 to 21 months old, however, the Male Excess offspring was significantly less than its Control cohorts. From 15 to 21 months of age, poor male offspring had intermediate weights of the body and does not differ significantly from its Control and Excess cohorts. It also shows that there were no significant differences in the diet group in weights of the adult female infants' body from 3 to 12 months old. 15 to 21 months old, however, the female Excessed offspring was significantly less than its Control and Deficient cohorts. From 15 to 21 months of age, poor female offspring was more curious than their control cohorts, but not significantly. Using only animal data that died before the completion of the study at 21 months of age, we analyzed death weights for group differences. There were no significant differences in diet group in death weights. There was a significant difference of sex with women who weighed less than their male cohorts at the time of death: F(1, 28)=5.025, p=0.033. There was no significant interaction with Diet-by-Sex. Although there was no importance Differences of the diet group, a tendency was observed to decrease the weights of death for both men and women in the group Excess (Control=598±55g, Deficient=543±50g and Excess=499±37g for men; Control=480±46g, Deficient=466±57g and Excess=396±30g for women). The mummary adenomas were rare in our Wistar rat colony. Only two Sufficient, an Excess and a Control descendence had such tumors. Seizures were rare with only four deficient descendants and one exhibiting seizure activity. Seizures always occurred at the end of their useful life and were associated with anemia and kidney failure as evidenced by the skinny appearance of the animal, necropsies and blood analysis. The animals of the three groups had the same findings of the necropsy. Neocropsies indicated chronic kidney diseases as evidenced by chronic interstitial glomerulonafrapathy (enlarged glomeruli, thick glomerular capsules and basal membranes), thick tubular tubular tubular membranes, tubular collapsing ducts, proteactic tubular tubular chestnuts, dystropic mineralization, interstitial infiltration Consistent with kidney disease, blood analysis showed abnormally high levels of nitrogen of blood urea (BUN), creatinine and phosphate. Livers generally had macrovesicular and microvesicular multifocal lipids, portal fibrosis, periportal lymphoid infiltrates, micorgranulomas and microaccesses. Consequent to liver disease, blood tests showed abnormally low albumin and hypoproteinemia, as well as abnormally high transamine levels. Other blood outcomes included abnormally high white blood cell counts, glucose and cholesterol levels, as well as abnormally low hematocrite, red blood cell count and hemoglobin. Some lungs had severe chronic histiocytic pneumonia (aveolar pryophagos, lymphocytic infiltrates). 3.4. The ABR thresholds (hearive hole) show the ABR thresholds as group functions of Diet and Frequency of Tone Pipe. There was a significant effect for the Diet group: F(2, 52)=4.293, p=0.019. Couple comparisons showed that the Excess group had significantly higher ABR thresholds than the Control group in tone pip frequencies of 2, 4 and 16 kHz (pMean±SEM ABR thresholds as diet group functions and tone frequency. Excess downwards had raised (the worst) thresholds than their Control and Deficient cohorts: *Different Excess from Control; † Different Excess from Control and Deficiency; **Excess and Deficiency Different from Control. shows ABRs series produced by 16 kHz tono pips of low intensity stimulus of the depictive offspring of old descendants in the Control, Deficient diet groups. Control (panel A) and poor animals (panel B) had their ABRs disappear below 25 and 30 dB respectively, while the animal Excess (panel C) had an ABR at 40 dB but not at 30 dB. Therefore, the animal Excess had raised the ABR threshold (defined technically as ≥2 standard deviations above the mean of the Control group), indicating hearing loss from ~10 to 15 dB. Serial ABRs promoted by 16 kHz tone pips of offspring stimulus intensities of the representative offspring of older adults descendants in Control, Deficient ω-3 FA and Excess ω-3 FA diet groups. ABRs had ABRs in 25 and 30 dB, respectively. On the other hand, the Excess descent had an ABR at 40 dB but not at 30 dB, indicating a high ABR (worse) threshold of ~10–15 dB.3.5. ABR Latency Intensity Profiles (L-I) The L-I profile can diagnose whether a subject's hearing loss is CHL or SNHL. shows the P2 wavelength L-I profiles of ABR produced by the 16 kHz tone pp condition for the seven animals (46.7%) in the group Excess with abnormal ABRs. The 16 kHz condition was chosen because age-related hearing loss is typically a high frequency hearing loss. The shaded region is the range of normality derived from control data. This figure shows that the L-I profiles of five Excess descendants were normally within normal limits in the higher stimulus intensities, but fell above the normal limits in the lower stimulus intensities. Therefore, these five children of Excess had L-I profiles consistent with SNHL, although soft SNHL. Therefore, 5 out of 15 Excess breeds (33.3%) had some degree of SNHL. In contrast, only 1 of 23 Control (4.3%) and 1 of 22 Deficiencies (4.5%) showed SNHL patterns: Chi Square (2)=9.111, p=0.011. Six of the Excess animals also had high ABR thresholds, defined as ≥2 standard deviations (SD) above the Control group means [].ABR latency intensity profiles (L-I) produced by 16 kHz pips tone of the seven descendants of Excess with abnormality. The shaded region is the normality range for the P2 wave latency (mean±2 SD) derived from the Control group data. Five of these children of Excess had L-I profiles that fell above the normality range in the lower stimulus intensities, suggesting sensorineural hearing loss (SNHL). Six of Excess breeding did not have ABR at the lower stimulus intensity levels. 3.6. ABR (A-I) Intensity Profiles As young adults, the Excess descent showed a relatively small A-I pattern of ABRs in the lowest stimulus intensities and relatively large amplitudes in the higher stimulus intensities []. To investigate whether this pattern persisted in adulthood, we measured ABR P2-N2 amplitude as an index of ABR's amplitude for reasons described in the Methods section. compares the A-I profiles for the P2-N2 wave in the three age categories. shows the A-I profiles for the old adult offspring caused by the 16 kHz tone pip condition. The 16 kHz pp condition was chosen because it was the most sensitive to group differences. There were no significant effects for the Diet group or for the Diet-by-Sex interaction. There was a significant effect for Sex, indicating that women had greater ABR amplitudes than men: F(1, 54)= 8.986, p=0.004. There was a significant effect for the intensity of the tone pipe, indicating that the amplitudes decreased as the tone pip intensity decreased: F(5, 270)=647.880, p=0.000. There were no significant interactions with the intensity of tone and sex or diet. Although there were no significant differences in the Diet group, the Excess group in old age showed a similar profile of A-I seen when they were young adults. In other words, the old breed of Excess adult had large amplitudes of ABR in response to the higher stimulus intensity (p=0.052, see). ABR (A-I) range intensity curves for offspring in the three diet groups such as puppies (A), young adults (B) and old adults (C). In the three ages, the breeding of Excess had abnormal growth in its ABR amplitudes (the P2-N2) as the stimulus intensity increased, suggesting hyperacusis. Tone pip stimuli=4 kHz (pups), 8 kHz (old adult) or 16 kHz (older adults). *Excess greater than Control and Deficient at p3.7. Maximum ABR expansions For the maximum amplitude of ABR, we evaluate the largest waveform (the P2-N2) wave caused by our highest stimulus intensity of 100 dB. These amplitudes mean ±SEM caused by the tone pyps of 2, 4, 8 and 16 kHz for control, the deficient offspring and the extase are presented in . Despite the old Excess offspring having the largest ABR amplitudes in the four tone pip frequencies, there were no significant effects for the Diet, Sex or Diet-by-Sex interaction. There was a significant effect for the Frequency of the Tone Pipe: F(3, 159)=27.149, p=0.000 and for the Frequency-by-Sex interaction: F(1, 159)=3.803, p=0.019 but not for any other interaction with the Frequency variable. As seen in , the ABR amplitudes were larger in response to the 8 kHz tone pip condition, smaller to the 2 kHz condition. Table 1The P2-N2 amplitude of ABR (μV) as dietary group functions, descending age and tone pip frequency (mean±SEM) Pip frequency of single Diet Group and Age2 kHz4 kHz8 kHz16 kHzA. (n=45)3.2±0.14.3±0.24.9±0.25.4±0.2 Deficiency (n=50)3.5±0.14.3±0.25.1±0.25.4±0.2 Excess (n=42)3.8±0.1 (PND 174) Control (n=44)3.5±0.13.9±0.2 We evaluate the ABR amplitudes of our animals first as young adults [] and now as old adults. We do not intend to evaluate the amplitudes of ABR for our publications by describing our animals as puppies of 24 days [,]. To see if the larger ABR amplitude pattern existed in the Excess offspring when they were puppies, we decided to analyze the pup data. The results were consistent with our young adult findings [] and older adults (present study). Specifically, the P2-N2 amplitudes of the ABR caused by the highest tone pip intensity (100dB) were larger in the Excess offspring. There was a significant effect for the Diet group: F(2, 131)=3.198, pThe Excess puppies also showed the same pattern A-I seen when they were young adults and old adults. That is, the puppies of Excess had an abnormal growth in their P2-N2 amplitudes as the intensity of the tone pipe increased. shows this pattern of growth of excess amplitude in the Excessed offspring in the three age categories. For pup data, there were significant effects for tone pipe intensity: F(6, 864)=1744.300, p3.9. For neuronal transmission times (P4 delay), we evaluate the ABR produced by the highest stimulus intensity of 100 dB. There were no significant effects for the Diet, Sex or Diet-by-Sex interaction. For example, the Control, Deficient and Excess descent had a similar average of 3.96±0.04, 3.94±0.04 and 3.99±0.05 ms when the data of the four-tone stimulus conditions were combined. There was a significant effect for the Frequency of the Tone Pipe, indicating that the P4 latency gradually became shorter (more rapid) as the frequency of the tone pipe progressed from 2 to 16 kHz: F(3, 156)=45.955, p=0.001. No significant effects were found on the diet or sex for the secondary outcome variables of the afternoons P1, P2 or P3 or P1–P4 IPL.3.10. Temperatures, weights and ages during ABR recordings There were no significant differences in body temperature during ABR recording sessions for Diet, Sex or Diet-by-Sex interaction. The respective average temperatures ±SEM for control, deficient and excess groups were 37.7±0.08, 37.6±0.09 and 37.5±0.10 °C. There were no significant age differences during ABR recording sessions for the Diet, Sex or Diet-by-Sex interaction. The respective mean ages ±SEM for the Control, Deficient and Excess groups were 482±3, 483±3 and 474±3 days old. There were significant differences in body weights during the ABR recording sessions for the Diet group: F(2, 54)=4.620, p=0.014 and for Sex: F(1, 54)=59.963, p=0.000 but not for the Diet-by-Sex interaction. The respective weights of the medium-sized body for the males Control, Deficient and Excess were 754±92, 779±55 and 696±114 grams. The weights of the average body ±SEM for women of control, deficiency and excess were 579±87, 613±60 and 581±82 grams.4. Discussion As hypothesized, the overnutrition of maternal AFA ω-3 during pregnancy and breastfeeding (excellence group) shortened the lifetime of adult offspring. The group Excess showed an increase in the appearance of presbicusis (aging-related sensory hearing loss), a persistence of two sensory/neurological abnormalities from the weaning and young adulthood to the old adult age in the form of high ABR thresholds (hearive loss to soft sounds) and hyperacusis (strong neuronal inhibition to sound). Excesses of offspring weighed less than their cohorts Control and Deficiency in old age, despite the three groups that weighed the same in young adulthood. Contrary to our hypothesis, the maternal deficient condition ω-3 FA had no effect on descending life, and the Excess and Deficiency groups did not show a greater prolongation of the neuronal transmission times in old age. Although Barker's hypothesis predicts the shortening of life as a result of an adverse fetal and/or neonatal environment, this parameter is rarely studied. A small number of animal studies have found shortened life span resulting from prenatal drug [,], hypoxia [] and malnutrition proteins []. This study is the first to report a shorter life of adult offspring of excess maternal diet ω-3 FA during pregnancy and breastfeeding. Deficient control and reduction of the useful life of the data are favorably compared to the normative data, while our Excess offspring was significantly abnormal. Open-race Wistar rats have a medium life of ~760 days []. On the other hand, our Excess offspring had an average life span of 506 days and an average life period of 524 days, for an estimated reduction of 31-33% in life. This is great compared to the reductions of 3-22% of life caused by other prenatal exposures [,,,,]. Excessive offspring had only 28% survival at 640 days when our study was completed. In contrast, control and poor offspring had survival rates of 68 per cent and 67 per cent to 640 days. Women had better survival statistics than men, which match literature []. Too much and deficient offspring had normal birth weights, but they were underweight by the peripheral age of 24 days []. Weight differences dissipated by the young adult age of ~5.5 months []. The current study found that Excess and deficient offspring continued to have normal body weights before the age of 15 months, when Excess offspring began to lose weight. Necropsia indicated that kidney failure was the main cause of death in the three dietary groups. Kidney failure is a common cause of death in old rats []. Therefore, it was not unusual that most of our Excess offspring died of this disease. However, it was unusual for our daughter of Excess to contract renal insufficiency at a much younger age than poor control and offspring. We don't do a detailed investigation of kidney failures. Therefore, we do not know whether kidney failures were secondary to infection, diabetes, hypertension, fewer cellular numbers, content and fospholipid function of abnormal kidney membrane, or some other cause. It would be important for future studies to investigate this issue because it would guide the management of patients in negatively affected children. In addition to kidney disease, some animals exhibited liver, lung, and blood chemistry. Such findings are common in old rats []. However, it would be important for future studies to determine the pathogenic basis of these pathologies and whether the excess of NIC-3 FA (or imbalance) during pregnancy and breastfeeding leads to the early appearance of these disorders. Our Wistar rats had a low incidence of mammal adenomas. This contrasts with Long-Evans rats that have a high incidence in old age []. A few descendants of Excess experienced seizures in old age, probably secondary to complications of kidney and liver disease. The excess of offspring had ABR thresholds significantly higher than that of Control, which indicates a lower hearing acuity to low intensity stimuli. Poor offspring had ABR thresholds that were intermediate to Excessity and deficient offspring. Similar results occurred in these animals as peripheral puppies [] and young adults []. Thus, there was a persistence of this abnormality throughout his life. Test ages and body temperatures were similar in diet groups during ABR recordings, eliminating these variables as confusing variables. The ABR L-I profiles indicated that 33.3 per cent of the Excess offspring had sensorineural hearing loss. This condition was not present when they were puppies [,] or young adults []. Thus, they developed this condition in old age. The beginning of SNHL in old age is called presbicusis. We saw this condition in less than 5% of the animals of Control and Deficiency. Therefore, some children of Excess had an early start or a higher risk for this age-related hearing disorder. This finding is consistent with our hypothesis of an improved sensory/neural degeneration in the old age. Other Excess offspring had raised ABR thresholds (ear loss) without SNHL. We speculate that these last animals had a poor neuron synchronization or a small number of neuronal units shooting in response to low-intensity sound stimuli. Any situation would cause a reduction in ABR amplitudes in response to low-intensity stimuli and poorer ABR thresholds [].As puppies () and young adults [], our Excess offspring showed another ABR anomaly. Specifically, they had raised the ABR thresholds in response to the lower tone pip intensities but abnormally large ABR amplitudes in response to higher intensity. Such pattern is consistent with a pathological condition known as hyperacusis. Hyperacusis is due to impaired neural inhibition []. As older adults, our Excess offspring showed a persistence of this pattern. It is true that the ABR hyperacusis evidence in the old Excess offspring was not strong enough to achieve statistical significance. The failure to achieve statistical importance was due to smaller group sizes, the development of presbicusis in several Excess breeds, and some of those most affected by the Excess of the calves who had died before their ABR test session. A deficiency for our study was the size of the population. We had to stagger our rat colony because of the decline in financial resources. This was further complicated by the unexpected and rapid death of Excess' offspring. Our study originally programmed ABR testing animals at 18 months (~550 days) of age because previous prenatal exposure studies indicated improved treatment effects in ABR [,] and minimum mortality rates at this age []. Consequently, we had to reschedule the ABR tests at ~16 months old, but for this time the Excess group was up to 60% of its population. Despite this loss of population and statistical power, the effects of the Excess treatment were strong enough to cause significant effects in life, body weight, ABR thresholds and presbicusis, as well as to indicate a persistence of hyperacusis. Another deficiency was the lack of strong effects on poor offspring. Evidently their mothers had adequate nutritional stores to compensate for the dietary deficiency ω-3 FA. In retrospect, we should have placed these dams on your treatment diet several weeks before appearing in an attempt to exhaust your ω-3 FA stores. A high omega-3 FA diet for a rat pregnancy study has precedence and relevance. A study by the American Nutrition Institute recommended that pregnant and nursing rats receive the AIN-93G diet containing 7% of oil []. Like our study, other studies of pregnancy and breastfeeding of rats have used diets containing fish oil of 7% or higher [,]. Our Excess condition had no significant effects on birth variables [,]. On the other hand, in some human studies [, -,] and rats, a reduction in birth weights was observed after high consumption of marine oil during pregnancy. Thus, our condition of Excess was less fetotoxic. It is recommended that preclinical and reproductive toxicology studies use a wide range of ratios ω-3/ω-6 FA [] and a high dose that is 10 to 100 times the human dose to test the drug safety factor, to compensate for the different nutritional needs of the rat or the insensitivity to the medications, and to reveal unspecified morbidities at lower dose levels []. Large doses are consumed voluntarily by [,,,,,] or are administered clinically to pregnant women to reduce premature deliveries and promote child development [,] and are administered for child therapy []. The current study was a preliminary study designed to explore what lifelong morbidities are possible under extreme conditions. Future studies should evaluate more moderate doses and levels of down tissue peroxide to better determine how much dietary fish oil is too much. The 3 diets had polyunsaturated fatty acids (PUFA). Hence some oxidation is inevitable in studies like ours. It is unlikely that the oxidation of diets during maternal consumption was a confusing variable, however. The addition of the TBHQ condom substantially inhibits oxidation [] and is a standard ingredient in PUFA rolling diets [,,]. We use standard procedures to store diets in hermetic bags in a darkened cooled room and replace them twice a week to protect against oxidation. The lower growth in the Excess offspring occurred postnatally, not prenatally. Therefore, it is likely that an important factor in its postnatal growth delay was breast milk consumption, which had significantly high levels of NIC-3 FA and low levels of ω-6 FA compared to Control []. When women received 200 mg of DHA daily from gestation week 21 until the third month of breastfeeding, their babies had less body weight []. With this small amount of DHA and lack of opportunity for oxidation, reduced postnatal weights cannot be attributed to oxidation effects. Finally, there is the precedence that a nutrient-rich maternal diet can be harmful. Although beneficial in moderation, the over-supply of prenatal vitamin A, iron [] calories and fat [,] have harmful effects on offspring. Also, FA NIC-3 in moderation is beneficial for fetal and neonatal development, but the increase in evidence indicates that its excess support may be harmful. The current study is the fourth of a series of articles that describe the progress of our animals throughout their lives, first as puppies [,] then as young adults [] and now as old adults. To summarize these articles, we find a mixture of transient, permanent, recurring and adult effects: (A) As a transient effect, the Excess' offspring showed neuronal transmission times delayed as puppies [] but not as young adults [] or as old adults. (B) As permanent effects, the Excess offspring showed elevated ABR thresholds (hearing loss) andnormally large ABR broades in response to high stimulus intensities (hyperacusis) as pups, as young adults [] and as old adults. In a related study, we also find a permanent change in fatty acid profiles of the body of the breed Excess in that its relationship ω-3/ω-6 FA was significantly greater than its Control and Cohorts deficient as adults []. (C) As a recurring effect, Excess' offspring had low body weight as weaned puppies [], normal weights like young adults [] and a recurrence of low body weights as old adults. (D) As adult-onset effects, the Excess offspring had a shortened life possibly span caused by early-onset kidney and liver failure and a significant proportion of the Excess offspring had an early inset or increased risk for presbycusis. Deficient offspring were often normal or intermediate between their control and excess cohorts in these result variables. There are several mechanisms by which the excess of ω-3 FA or the imbalance ω-3/ω-6 FA could have produced adverse effects on our offspring: (A) Relative excess of ω-3 FA will reduce AA concentrations in blood, brain, and other tissues by competitive displacement []. Reduced AA concentrations affect the growth of the fetal and the baby [,] and alter the cell membrane, organ, brain and sensory functioning []. As a result of this notion, our Excessive offspring had decreased body composition levels AA as adults []. (B) Excess ω-3 FA consumption can cause oxidative stress and subsequent cell apoptosis []. (C) Malnutrition can cause epigenetic and hormonal changes in the health disorders of the fetus and adults [,,,,]. (D) The oocyte's exposure to a high environment in ω-3 FA results in disturbed mitochondrial distribution, metabolism and calcium levels, negatively affecting embryonic morphology and development []. (E) A diet rich in ω-3 FA can decrease milk performance of lactating rats [] and alter the composition of fatty acids of breast milk [], which leads to a delay in postnatal growth. It may seem disconcerting that some studies report beneficial effects of the supplementation of the pre- and postnatal FA ω-3, while others report harmful effects or even not. Studies that report beneficial effects generally focus on visual functions [,] or cognitive [,]. Studies that report adverse effects generally focus on auditory functions [–,,] or engine [,,]. One possibility is that the excess or imbalance of FA NIC-3 has differential effects, benefiting some functions by harming or not influencing others []. Other explanations relate to differences between doses and dependence and population in metabolism: (A) Harmful effects are associated with ω-3 FA megadosing or with a ω-3/ω-6 FA imbalanced relationship. b) Populations that consume high levels of marine oil for generations may have adapted to these diets and are less likely to adversely affect them. In conclusion, this is the first study to investigate the long-term consequences of the overnutrition of maternal NIC-3 FA. We found that a maternal diet rich in ω-3 FA, when taken during pregnancy and breastfeeding, had permanent harmful effects on the lifespan of the offspring and hearing function. These effects were probably due to a nutritional toxicity that programmed offspring for lifelong health disorders. As others emphasize, caution should be exercised when taking too much ω-3FA or a high relationship ω-3/ω-6 FA during pregnancy, breastfeeding and feeding child formulas [,,,,]. Children who are prenatally or post-natally exposed to excess of FA ω-3 or a high proportion of FA ω-3/ω-6 should be evaluated and administered for possible sensory, neurodeveloped and adult health disorders. These children may be at risk for hearing-processing disorders (APD), leading to the comorbidities of speech and language delays, learning disabilities and attention deficit disorders. There is provisional evidence of a decrease in verbal skills in these children [,]. They may also be at risk of presbicusis, hypertension, type II diabetes, early kidney and liver failure, and shorter life periods in adulthood. Therefore, it is necessary that these children continue in the long term, study the pathogenic bases of these disorders and implement intervention strategies. Recognition With the support of scholarships from the Gerber Foundation and the National Institute of General Medical Sciences (GM58905), none of which contributed to the design, analysis or writing of this study. We thank the Dr. Karen Rossman, DVM (Lab Animal Resources Department, Wayne State University) for collecting and analyzing tissues and blood samples. FootnotesDescargos de Editor: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will be submitted to copy, composition and revision of the resulting test before it is published in its final citable form. Please note that during the production process you can discover errors that could affect the content, and all legal claims that apply to the journal. Conflicts of interest There were no conflicts of interest. ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA

5 Reasons You Should Not Take Too Much DHA During Pregnancy? HealHealth.in

DHA & Helping Baby Develop a Healthy Brain - Mommy's Bundle

DHA and Pregnancy: What You Need To Know

Taking DHA (Docosahexaenoic Acid) in Pregnancy: Risks & Benefits

DHA & Pregnancy - Why This Omega-3 Is Important For Mother & Child

5 Ways Pregnant Women Can Get More DHA in Their Diets | OmegaQuant

DHA and Pregnancy: What You Need To Know

DHA and Pregnancy: What You Need To Know

You're Pregnant! Now What? | OmegaQuant

DHA and Pregnancy: What You Need To Know

DHA Supplementation During Pregnancy: How Much is Enough?

DHA Supplementation During Pregnancy: How Much is Enough?

Fish Oil During Pregnancy - OmegaVia

DHA Supplementation During Pregnancy: How Much is Enough?

DHA and Pregnancy: What You Need To Know
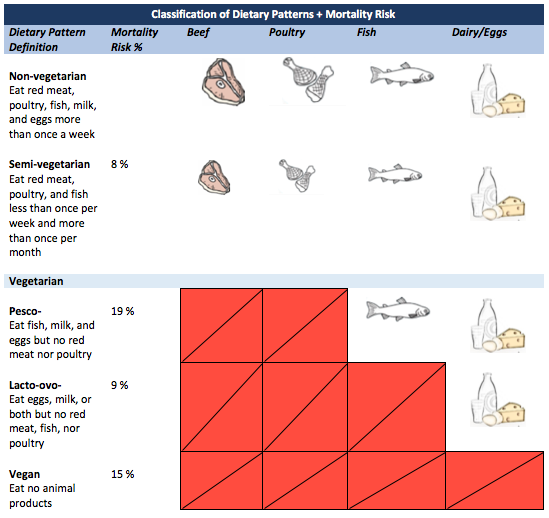
DHA Supplementation During Pregnancy: How Much is Enough?
/78405778-56a76e8c3df78cf77295e4cd.jpg)
Do Pregnant Women Need to Take DHA Supplements?

DHA and Pregnancy: What You Need To Know
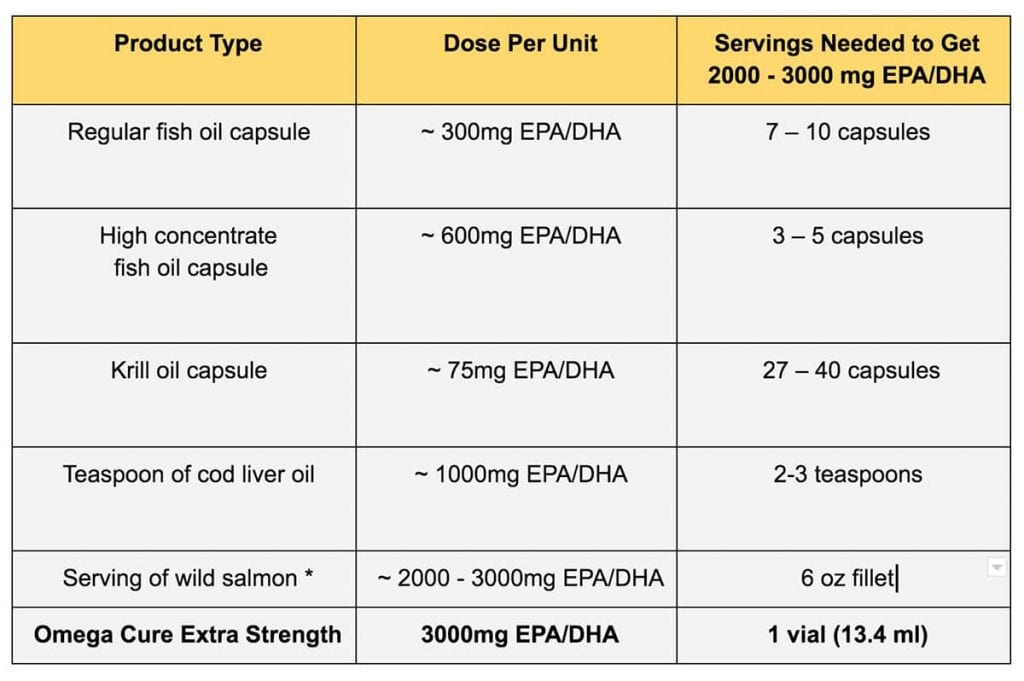
How Much Omega-3 Fish Oil A Day Will Produce Results?
:max_bytes(150000):strip_icc()/GettyImages-92792399-56aa070c3df78cf772ac12ba.jpg)
DHA Health Benefits in Pregnancy

DHA and Pregnancy: What You Need To Know

Fish Oil During Pregnancy - OmegaVia

Pin on Pregnancy and maternity infographic
Taking DHA (Docosahexaenoic Acid) in Pregnancy: Risks & Benefits

DHA Supplementation During Pregnancy: How Much is Enough?

You can't rely on fish oil supplements in pregnancy to make your children smarter

Do Pregnant Women Need to Take DHA Supplements?

DHA & Pregnancy - Why This Omega-3 Is Important For Mother & Child

Best DHA Supplement for Pregnancy - The Diet Standards Difference

You can't rely on fish oil supplements in pregnancy to make your children smarter

Omega 3 Fish Oil Supplements Pregnancy Guide | Bare Biology

DHA and Pregnancy: What You Need To Know

DHA & Pregnancy - Why This Omega-3 Is Important For Mother & Child

5 Ways Pregnant Women Can Get More DHA in Their Diets | OmegaQuant
/114476859bysharaffMomentGetty-56a11c1a5f9b58b7d0bbcca0.jpg)
Use of DHA Supplements in Breastfeeding Women
Taking DHA (Docosahexaenoic Acid) in Pregnancy: Risks & Benefits

DHA Supplementation During Pregnancy: How Much is Enough?

DHA & Pregnancy - Why This Omega-3 Is Important For Mother & Child
5 Ways Pregnant Women Can Get More DHA in Their Diets | OmegaQuant

Prenatal Nutrition: What Your Doc Didn't Tell You — Bend & Bloom Yoga
Posting Komentar untuk "too much dha during pregnancy"