citric acid to lemon juice
 How to Make Elderflower Cordial – Food in Jars
How to Make Elderflower Cordial – Food in JarsWarning: The NCBI website requires JavaScript to operate. Quantitative evaluation of citric acid in lemon juice, lemon juice and fruit juice products available commercially KRISTINA L. PENNISTON1 Department of Surgery, Division of Urology, Faculty of Medicine and Public Health, University of Wisconsin, Madison, WisconsinSTEPHEN Y. NAKADA1 Department of Surgery, Division of Urology, Faculty of Medicine and Public Health, University of Wisconsin, Madison, Wisconsin2 Department of Medicine, Faculty of Medicine and Public Health, University of Wisconsin, Madison, WisconsinROSS P. HOLMES3 Department of Urology, Wake Forest University School of Medicine, Winston-Salem, North Carolina Puroli Purtranow3 Department of Urology, Wake Forest University School of Medicine, Winston-Salo, Carolina Pursis Citrate is a natural inhibitor of urinary crystallization; achieving the concentration of therapeutic urinary citrus is a clinical objective in the medical management of calcium urolitiasis. When supplied as liquids, beverages containing citric acid add to the total volume of urine, reducing its calcium saturation and other crystals, and may improve the excretion of urinary citrus. Information on the citrus content of fruit juices and commercially available formulations is not widely known. We evaluate the concentration of citric acid of various fruit juices. Materials and methods The citrus acid content of 21 juice and juice concentrates available commercially and the juice of three types of fruits was analyzed using ions chromatography. ResultsThe lemon juice and lemon juice are rich sources of citric acid, which contain 1.44 and 1.38 g/oz, respectively. The lemon and lemon juice concentrates contain 1.10 and 1.06 g/oz, respectively. The content of commercial lemonade citric acid and other juice products varies widely, from 0.03 to 0.22 g/oz. ConclusionsThe lemon and lemon juice, both of fresh fruit and juice concentrates, provides more citrus acid per litre than the ready-to-use grapefruit juice, orange juice ready to consume, and juice of orange expressed from the fruit. The lemonade formulations ready to consume and those that require mixture with water contain ≤6 times citric acid, on an ounce base, lemon and lemon juice. INTRODUCTION Citric acid (2-hydroxy-1,2,3-propanetricar-boxylic acid) is a weak tricarboxylic acid that concentrates naturally on citrus fruits. In physiological pH, and to a lesser extent in the urine, there is mainly as a trivalent anion. Citric acid is often used as an additive to provide acidity and agrity flavor to food and beverages. Citrate salts of several metals are used to offer minerals in biologically available forms; examples include dietary supplements and medications. Among the fruits, citric acid is more concentrated in lemons and lemons, which comprise up to 8% of the weight of the dried fruit. An important source of in vivo citric acid results from endogenous metabolism in mitochondria through ATP production in the citric acid cycle. Gastrointestinal absorption of citrus acid from dietary sources has been associated with a modest increase in urinary citrus excretion. – Urinary citrate is a powerful inhibitor of urinary crystallization. The citrate is freely filtered into the proximal tubular of the kidney. About 10% to 35% of the urinary citrate is excreted; the rest is absorbed in various ways, depending on the urine pH and other intrarenal factors. Citrate is the most abundant organic ion found in the urine. Hipocitraturia, defined as The amount of diet-derived citrate that may escape in vivo conversion to bicarbonate is reportedly minor. However, a previous study reported increase in urinary citrus after 1 week in 4 ounces of lemon juice per day, diluted in 2 L of water, in the old stone with hypocitraturia. Two retrospective studies showed an effect on the ancients of lemon juice calcium stone and/or consumption of lemonade in urinary citrate, but a recent clinical trial showed no influence on the lemonade in urinary citrate. The U.S. Department of Agriculture Nutrient Database does not provide information on the content of food citric acid. Although the content of lemon juice citrus acid has been reported, there is no published information about the content of the citrus acid of negotiable beverages in the United States. Knowledge of the content of citrus acid of fruit juices and commercially available lemonade and lemonade products may be clinically applicable to patients requiring improvement or maintenance of concentrations of therapeutic urinary citrus. Several methods have been used to measure food citrus content, including polarographic, enzyme and ion-exclusion chromatography.– These techniques are subject to interference, can be relatively insensitive, and can be laborious. In the last decade, ional chromatography using suppressed conductivity has become the method of choice to measure organic acids and other anions in a variety of matrices. We have used this technique to measure citric acid in different fruit juices. MATERIAL AND METHODS All the products were obtained from local supermarkets in Madison, Wisconsin and Winston-Salem, North Carolina, and included fresh fruits, fruit juices ready to consume, lemon concentrates and lemon juice and crystallized lemonade formulations. 100% pure yarn. Samples of ready-to-use drinks and juice concentrates were taken directly from their packages. The crystallized lemonade formulations were mixed with water according to the directions of the package. Of the fresh fruits, the juice was extracted manually. The samples were diluted 1/5000 in water for analysis. Team Ion chromatography analysis was performed in a system consisting of a Dionex ED50 conductivity detector, a Dionex AS11-HC 2 ion exchange column × 250-mm with a guard column at a controlled temperature of 30°C, and a 2mm Dionex ASRS-ULTRA (Dionex Corporation, Sunnyvale, CA). Chromatographic conditions A gradient of 20 to 35 mM of sodium hydroxide was used for 10 minutes; citrate eluted to 7.6 minutes. The peak areas were related to those of a standard curve to quantify the concentration of citric acid. Statistical methods We compare the concentrations of citric acid of juice groups and juice products with a variance analysis (ANOVA). Pair comparisons were performed using less significant difference tests protected by Fisher. RESULTS P ValuesThe citrus acid content of various fruit juices and fruit drinks are listed in . Comparisons of the citric acid content of juices and drinks, by group, are shown in . Lemon juice and lemon juices expressed from fruits contained the most citric acid (48 and 46 g/L, respectively). There was no difference between lemon juice and lemons for citric acid content (P =0.35). The lemon juice and concentrate lemon was similar to the citric acid content (34–39 g/L). Grapefruit juice and orange juice of juice formulas ready to consume, 100% contained 25 and 17 g/L, respectively. There was no difference between the regular formulation of orange juice and its "light" counterpart. The orange juice expressed directly from oranges had a citric acid content lower than the orange juice ready to consume (P =0,003). Of the commercially available lemonade and lemonade formulations, which are usually formulated to contain 15% real juice or less, those that are ready to consume contain more citric acid than the powder mixtures that were prepared mixing with water according to package instructions (P =0.03). There was no difference between the light and sugar-free lemonades ready to consume and their regular counterparts (P =0.76) and no difference between the unlighted and sugar-free lemonade and the crystallized and powdered lemonade mixtures (P =0.21). Comparison of citric acid concentrations (g/oz.) of juices and juice products per group. Bars, where shown, represent SD for each group. The values above each bar are the medium content of citric acid (g/oz.) per group. Groups with the same letters are similar (p не0.03). RTC, ready to consume; reg, regular. DISCUSSION The medical management of calcium urolitiasis depends on manipulating the balance of crystal promoters against inhibitors. Nutritional therapy to the risk factors of an individual patient is a basic strategy for the prevention of kidney stone and an appropriate attachment for pharmacological therapy. A cornerstone of prevention is to achieve the proper dilution of urine by improving the volume of urine. This reduces the supersaturation of the urine, a necessary first step in crystal formation. A second objective in preventing the formation of calcium stone is to improve the concentration of crystal inhibitors. Of these, the cytrate is the most important clinically, as it can be manipulated by diet or pharmacological therapy or a combination of it. While 320 mg (1.67 mmol) urinary citrate in a 24-hour urine collection is considered the cut for the definition of hypocitraturia, some clinicians point to a 24-hour urinary citrate concentration of ≥600 mg (3.12 mmol), which is closer to the urinary excretion of cysts of healthy individuals and non-stone trainers. Hypocitraturia, if severe or persistent, usually requires pharmacological therapy in the form of potassium citrate, which improves urine pH and also quotes excretion. The identification and promotion of fluid consumption that add to the crystal inhibitor potential of urine is attractive, not only to promote fluid intake but to improve urinary citrus excretion. Citric acid is a natural organic acid present in multiple fruits and juices. Data on the citrus acid content of fresh fruit juices and commercially available fruit juice beverages can be useful for building nutritional therapy regimens for ancients of calcium stone. The lemon and lemon juice, both of fresh fruit and juice concentrates, provide more citrus acid per litre than the grapefruit juice ready to consume, orange juice ready to consume, and orange juice expressed from the fruit. These data coincide with those reported above. As lemon and lemon juice contains 38 and 35 mg potassium/oz, respectively, approximately the same grapefruit juice and about 60% orange juice (contained potassium obtained from the Department of Agriculture, Agricultural Research Service, USDA National Nutrient Base for Standard Reference, Release 19, Nutrient Data Laboratory Home Page: , accessed 07/02/2007), the ingestion of lemon juice or cicrédito The distribution of lemon juice or lemon in broad water or other liquid, consumed throughout the day, would also add to the volume of ingested liquids, which would result in an increase in urine production and a reduction in urine supersaturation. More research should determine the bioavailability of dietary citric acid from various sources and characterize the response to dietary citric acid in ancient renal stone that are hypocitraturic, as well as those that are normocitraturic. The impact of eating citrates on urinary concentrations among the ancients of calcium stone that consume different diets (e.g., high/vegetable fruit intake in the face of low/vegetable fruit intake; high meat intake v low meat intake), since dietary patterns are known to influence urinary citrate concentrations. ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA

Lemon Juice: Acidic or Alkaline, and Does It Matter?

The acidity of lemons and home canning - Healthy Canning

Sourness & Scurvy – The Chemistry of a Lemon – Compound Interest

Cooking With Citric Acid: Tips and Tricks | Epicurious

Pin on Good to Know

What Is the pH of Lemon Juice?

The acidity of lemons and home canning - Healthy Canning

What Is Citric Acid, and Is It Bad for You?

WHICH IS A STRONGER ACID VINEGAR OR LEMON JUICE?
.jpg)
aldawaa: Isolation of citric acid from lemon juice
Which acids are present in lemon? - Quora
:max_bytes(150000):strip_icc()/Citric-acid-GettyImages-535858824-58c9523a5f9b58af5c6bd584.jpg)
What Is Citric Acid and How Is It Used?
DETERMINING THE CITRIC ACID CONCENTRATION OF LEMONS VERSUS L by Ignatius Gim

Is Citric Acid in Your Food? Here's Why You Shouldn't Worry - Public Goods Blog

Sourness & Scurvy – The Chemistry of a Lemon – Compound Interest

8 Clever Substitutes for Lemon Juice

How to Extract the Citric Acid From Lemons

Citric acid | The Mole | RSC Education
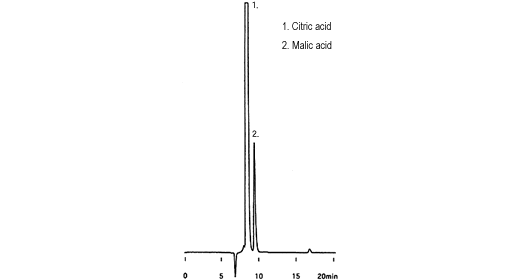
Organic Acids in Lemon Juice : SHIMADZU (Shimadzu Corporation)
.jpg)
aldawaa: Isolation of citric acid from lemon juice

Citric acid | Podcast | Chemistry World

Lemon juice improves the extractability and quality characteristics of pectin from yellow passion fruit by-product as compared with commercial citric acid extractant - ScienceDirect
Which acid is found in a lemon? - Quora
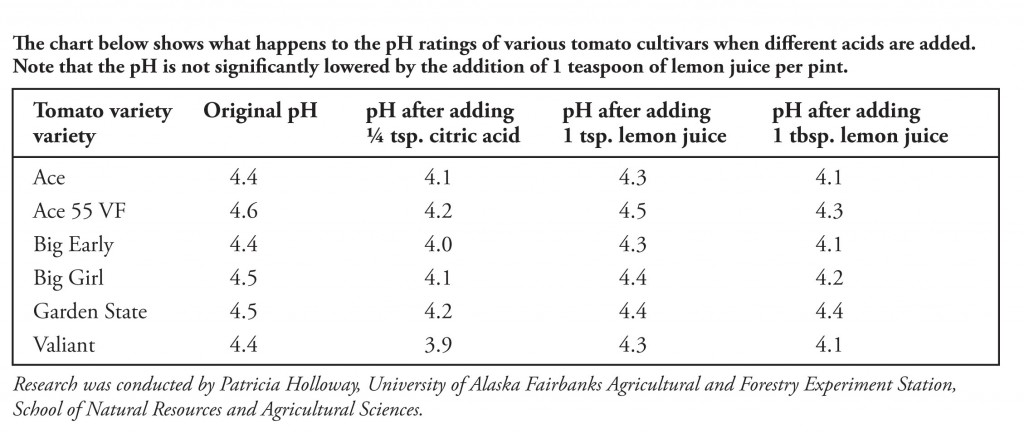
Citric acid and home canning - Healthy Canning
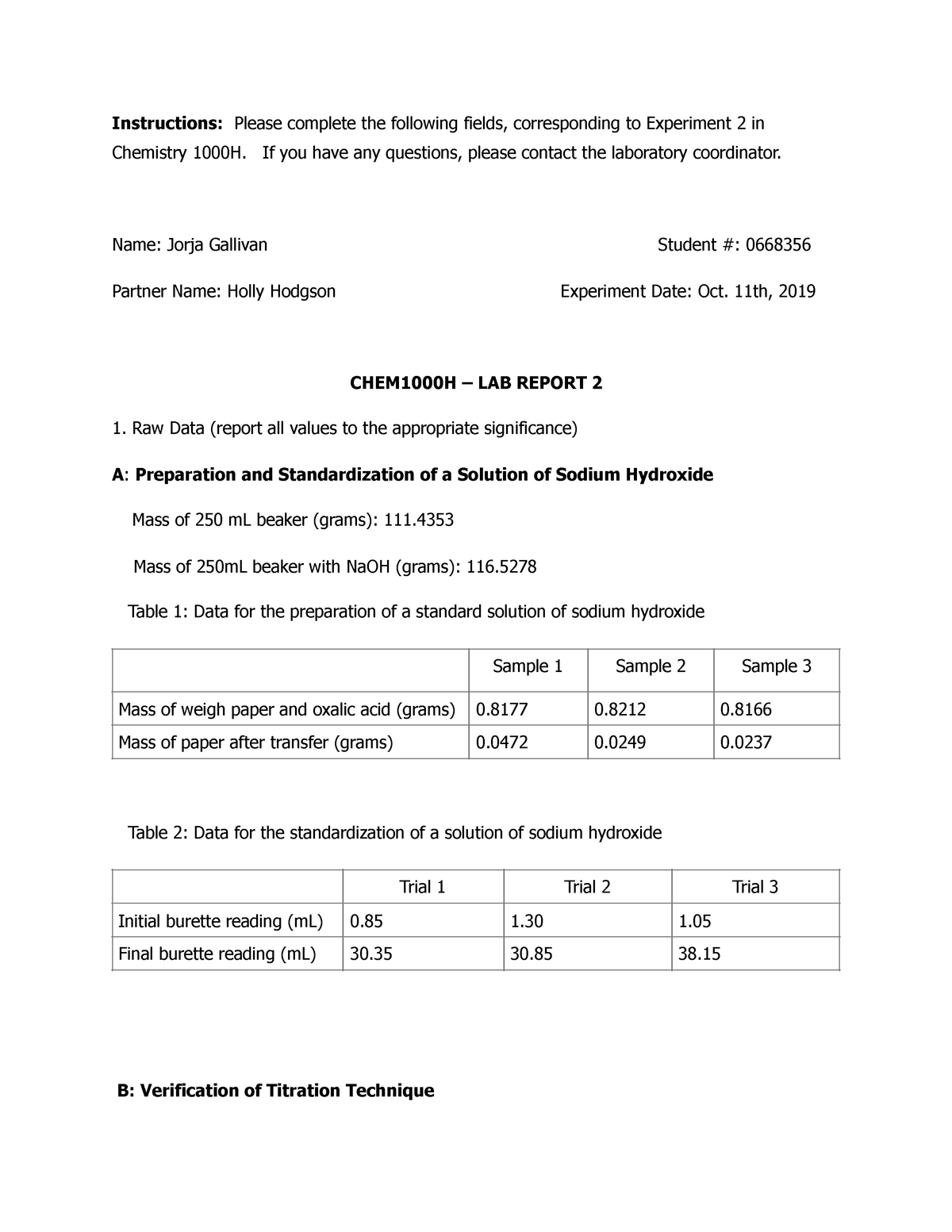
Determination of Citric Acid in Lemon Juice Lab - StuDocu

Cartoon Lemon, Lime, Citric Acid, Citron, Lemon Juice, Yellow, Citrus, Lemon Peel transparent background PNG clipart | HiClipart

4 Amazing Citric Acid Substitutes

Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice, And Commercially-Available Fruit Juice Products | Lemonade | Juice
/citric-acid-safety-cleaning-uses-1707021_05-ff88f42e69bd44b98866605ea4f57837.jpg)
How to Clean With Citric Acid

Passing the acid test: Lemon juice decreases blood glucose spike post-meal - Pokka study

Lemon Water for Acid Reflux: What You Should Know

Titration of lemon juice (Chemistry Laboratory Previews) - YouTube

Extraction of citric acid from lemon - Chemistry Stack Exchange

Lemon vs. lime: Differences in nutrition, benefits, and uses
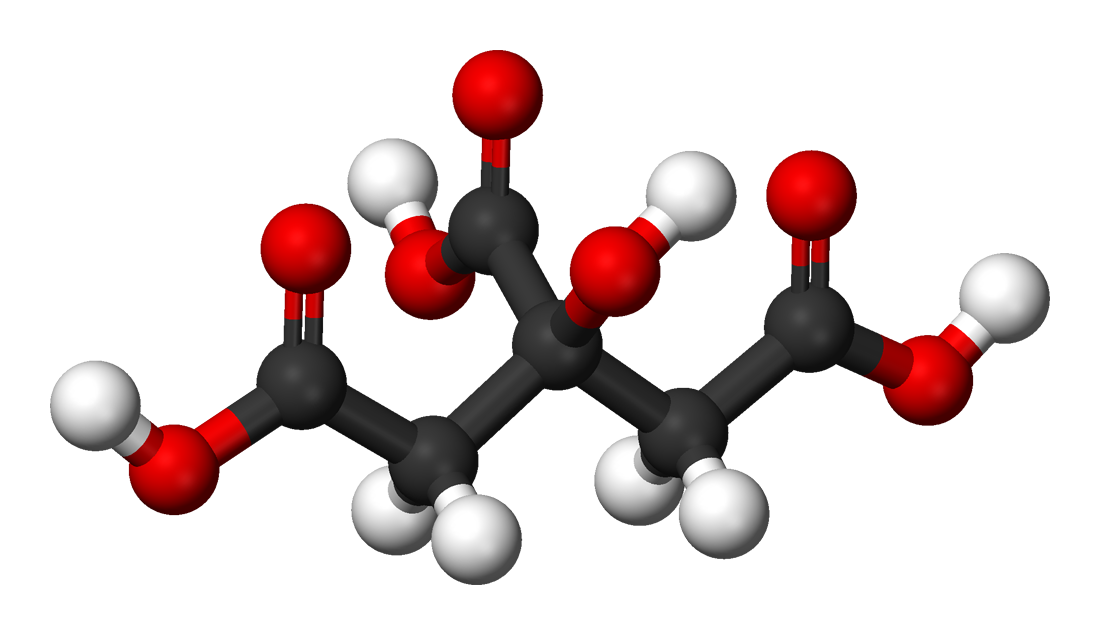
Citric acid - Wikipedia

9 Tasty Substitutes For Lemon Juice: From White Wine To Citric Acid - Boldsky.com

Solved: Chemistry-Titration, Molarity, Molar Ratio Hello :... | Chegg.com

Effect of lemon water soluble extract on hyperuricemia in a mouse model - Food & Function (RSC Publishing)

Lemon juice normally has a `pH` of `2`. If all the acid the lemon juice is citric acid and there are - YouTube
Posting Komentar untuk "citric acid to lemon juice"